
Previously, we briefly introduced Biomedical and Line DoD Technology Readiness Levels (TRLs) and discussed how a technology may develop through each stage of the TRL scale. Biomedical TRLs can be further categorized into TRLs for pharmaceuticals (drugs, biologics, and vaccines), medical devices, medical information management/information technology (IM/IT) and medical countermeasures (MCMs) following the FDA Guidance on Product Development under the Animal Rule. In this article, we will focus on biomedical DoD TRLs for medical devices.
Classification: Drug or Device?
Although the classification of products as either a drug or medical device can often be straightforward, for some products it is not so obvious. Some products that may intuitively be considered a drug may actually be classified as a device, and vice versa. In addition, some products do not fit exclusively into the category of drug or device but are instead combination products (e.g., drug-eluting stents, drug-containing patches). The classification of a product by the FDA is determined by its primary mechanism of action (PMOA). For example, heparin flushes were reclassified from drug to device in October 2006 as a result of a decision that heparin flushes exert their PMOA by physically occupying space and applying pressure within the catheter. The mechanism of heparin preventing thrombotic occlusions was determined to be a secondary function of the product. For more information, see the Review of the Processes for FDA Oversight of Drugs, Medical Devices, and Combination Products.
Medical Device DoD TRLs
Appendix E of the Department of Defense Technology Readiness Assessment (TRA) Deskbook details biomedical DoD TRLs, including TRLs for medical devices:
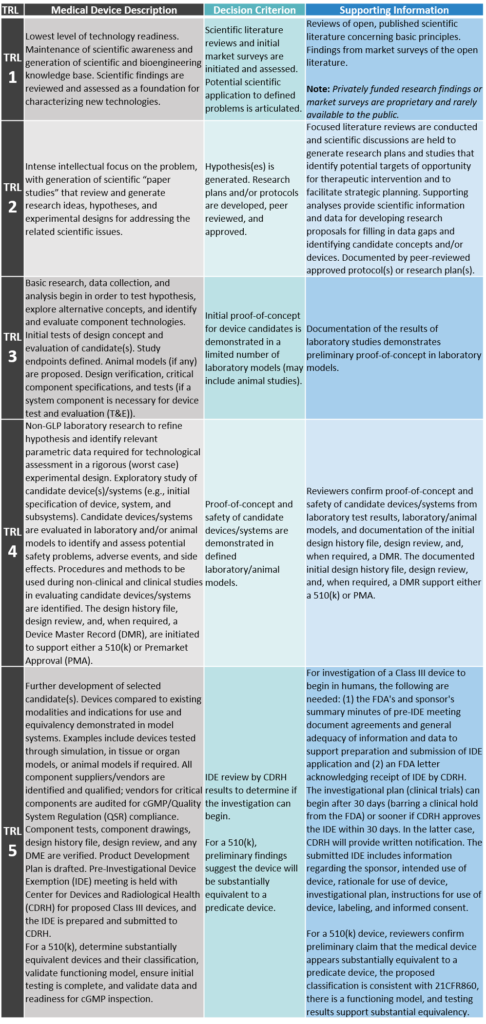
TRL 1: Basic Principles Observed and Reported
Lowest level of technology readiness. Maintenance of scientific awareness and generation of scientific and bioengineering knowledge base. Scientific findings are reviewed and assessed as a foundation for characterizing new technologies.

TRL 2: Technology Concept and/or Application Formulated
Intense intellectual focus on the problem, with generation of scientific “paper studies” that review and generate research ideas, hypotheses, and experimental designs for addressing the related scientific issues.

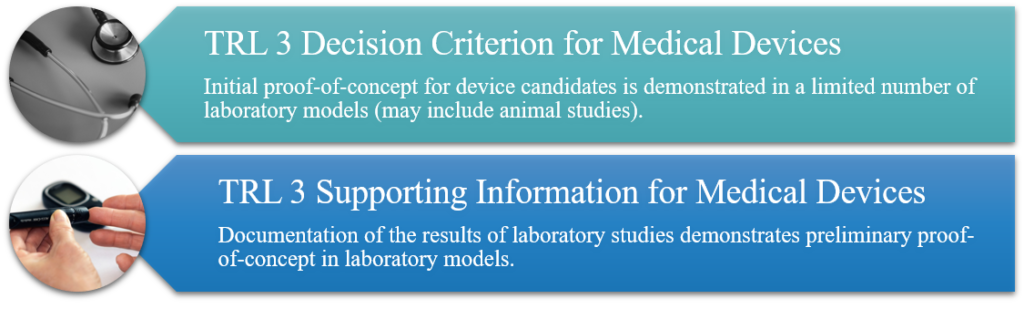
TRL 3: Analytical and Experimental Critical Function and/or Characteristic Proof-of-Concept
Basic research, data collection, and analysis begin in order to test hypothesis, explore alternative concepts, and identify and evaluate component technologies. Initial tests of design concept and evaluation of candidate(s). Study endpoints defined. Animal models (if any) are proposed. Design verification, critical component specifications, and tests (if a system component is necessary for device test and evaluation [T&E]).
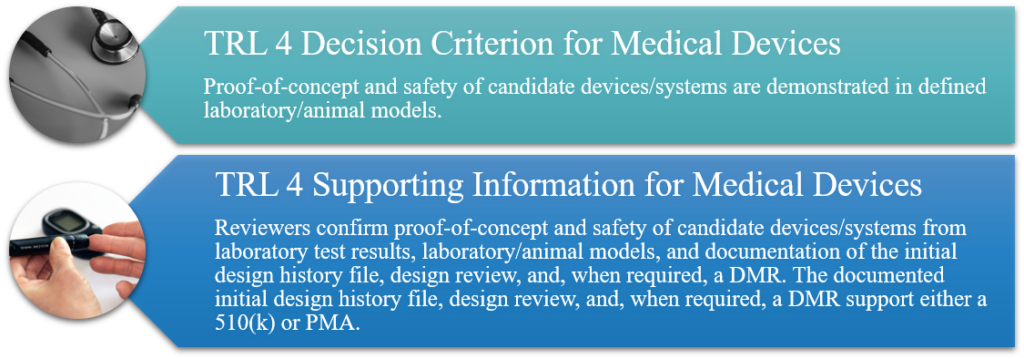
TRL 4: Component and/or Breadboard Validation in a Laboratory Environment
Non-GLP laboratory research to refine hypothesis and identify relevant parametric data required for technological assessment in a rigorous (worst case) experimental design. Exploratory study of candidate device(s)/systems (e.g., initial specification of device, system, and subsystems). Candidate devices/systems are evaluated in laboratory and/or animal models to identify and assess potential safety problems, adverse events, and side effects. Procedures and methods to be used during non-clinical and clinical studies in evaluating candidate devices/systems are identified. The design history file, design review, and, when required, a Device Master Record (DMR), are initiated to support either a 510(k) or Premarket Approval (PMA).
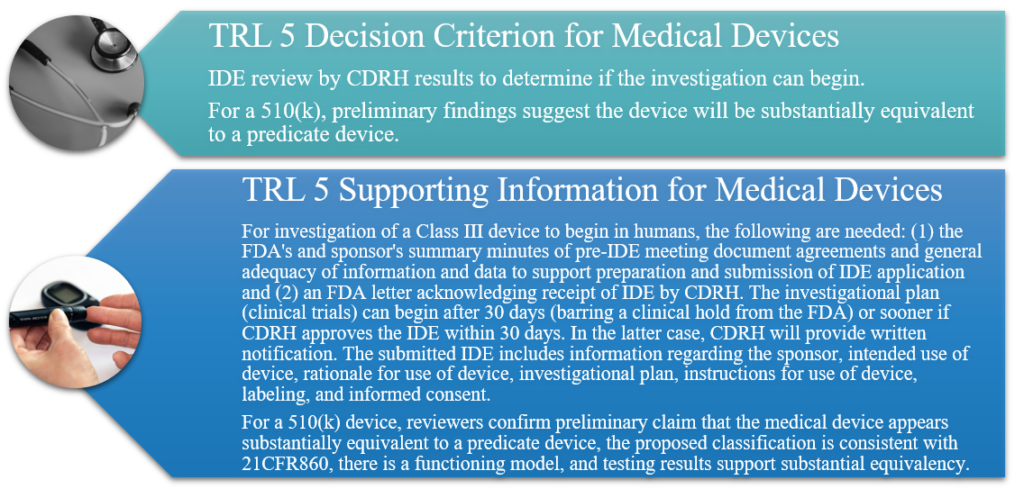
TRL 5: Component and/or Breadboard Validation in a Relevant Environment
Further development of selected candidate(s). Devices compared to existing modalities and indications for use and equivalency demonstrated in model systems. Examples include devices tested through simulation, in tissue or organ models, or animal models if required. All component suppliers/vendors are identified and qualified; vendors for critical components are audited for cGMP/Quality System Regulation (QSR) compliance. Component tests, component drawings, design history file, design review, and any DME are verified. Product Development Plan is drafted. Pre-Investigational Device Exemption (IDE) meeting is held with Center for Devices and Radiological Health (CDRH) for proposed Class III devices, and the IDE is prepared and submitted to CDRH. For a 510(k), determine substantially equivalent devices and their classification, validate functioning model, ensure initial testing is complete, and validate data and readiness for cGMP inspection.
TRL 6: System/Subsystem Model or Prototype Demonstration in a Relevant Environment
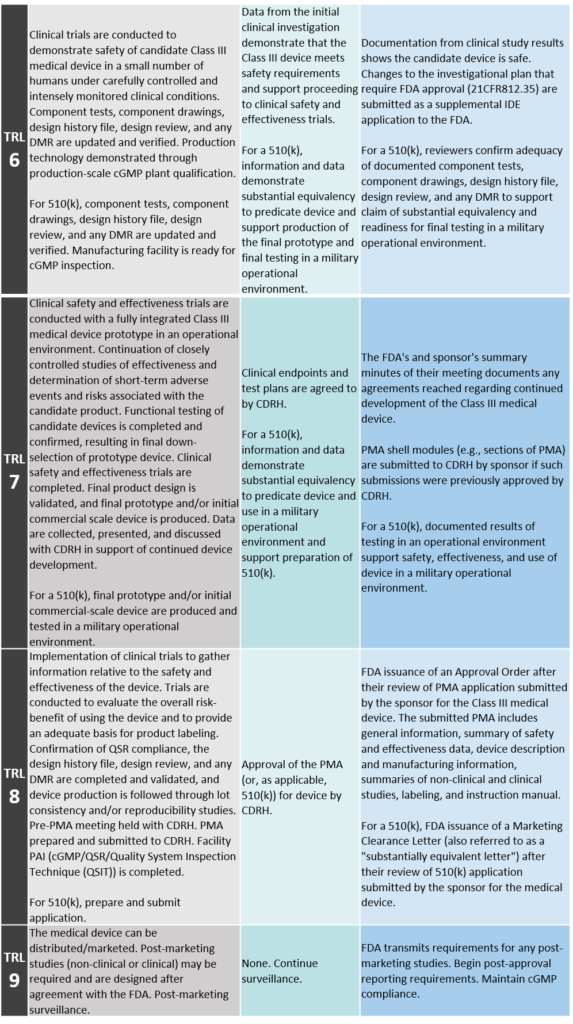
Clinical trials are conducted to demonstrate safety of candidate Class III medical device in a small number of humans under carefully controlled and intensely monitored clinical conditions. Component tests, component drawings, design history file, design review, and any DMR are updated and verified. Production technology demonstrated through production-scale cGMP plant qualification. For 510(k), component tests, component drawings, design history file, design review, and any DRM are updated and verified. Manufacturing facility is ready for cGMP inspection.

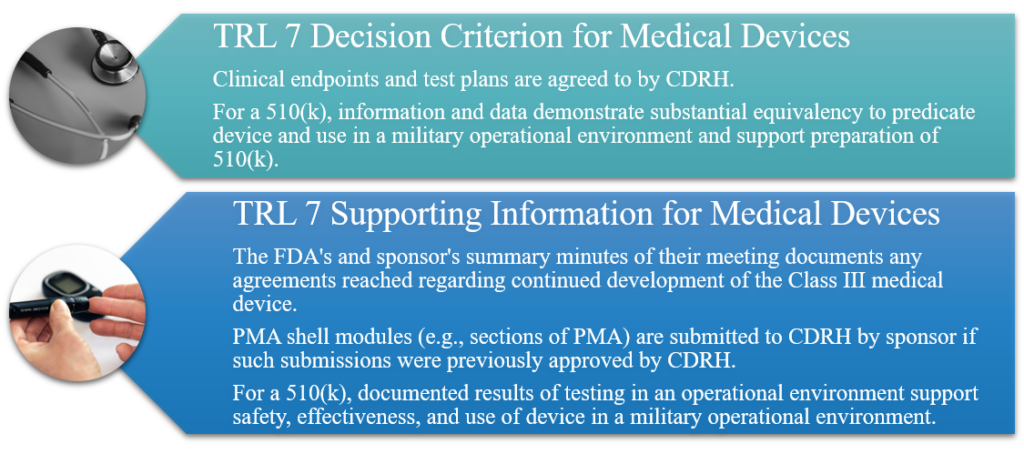
TRL 7: System Prototype Demonstration in an Operational Environment
Clinical safety and effectiveness trials are conducted with a fully integrated Class III medical device prototype in an operational environment. Continuation of closely controlled studies of effectiveness and determination of short-term adverse events and risks associated with the candidate product. Functional testing of candidate devices is completed and confirmed, resulting in final down-selection of prototype device. Clinical safety and effectiveness trials are completed. Final product design is validated, and final prototype and/or initial commercial scale device is produced. Data are collected, presented, and discussed with CDRH in support of continued device development. For a 510(k), final prototype and/or initial commercial-scale device are produced and tested in a military operational environment.
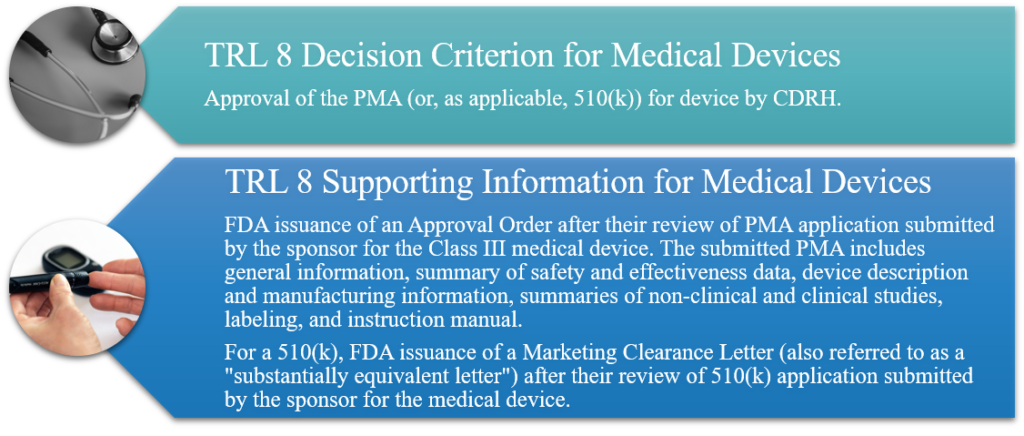
TRL 8: Actual System Completed and Qualified through Test and Demonstration
Implementation of clinical trials to gather information relative to the safety and effectiveness of the device. Trials are conducted to evaluate the overall risk-benefit of using the device and to provide an adequate basis for product labeling. Confirmation of QSR compliance, the design history file, design review, and any DMR are completed and validated, and device production is followed through lot consistency and/or reproducibility studies. Pre-PMA meeting held with CDRH. PMA prepared and submitted to CDRH. Facility PAI (cGMP/QSR/Quality System Inspection Technique [QSIT]) is completed. For 510(k), prepare and submit application.
TRL 9: Actual System Proven through Successful Mission Operations
The medical device can be distributed/marketed. Post-marketing studies (non-clinical or clinical) may be required and are designed after agreement with the FDA. Post-marketing surveillance.

Novel concepts and emerging technologies are generated from TRL 1 through TRL 3, during which device components and designs are identified and initial proof-of-concept is established. Applied research begins in TRL 4 with non-GLP safety and efficacy studies and continues into TRL 5, during which IDEs are prepared and submitted for Class III devices Investigation of Class III devices in clinical trials begins in TRL 6. Prototype maturation and demonstration continues into TRL 7 (Class III device design validated, and final prototypes produced) and TRL 8 (investigation of final Class III prototype in clinical trials and PMA/510(k) prepared, submitted, and approved). TRL 9 marks medical device production and distribution, with additional post-marketing studies and surveillance as required.
In future articles, we will discuss additional subcategories of biomedical TRLs (drugs, biologics, and vaccines; medical information management/information technology [IM/IT]), as well as how biomedical TRLs can be further translated with medical countermeasures (MCMs) that require animal rule/efficacy data. In addition, various TRLs are further defined for Biomedical and MCM applications, as well as TRLs developed or categorized by Federal Agency, and are available as resources at Tier Seven.
27 Apr
2018
