
Technology readiness assessments (TRA) are widely utilized by the U.S. Department of Defense (DoD) to determine a technology’s readiness for use in acquisition programs and projects (see the U.S. Government Accountability Office’s Technology Readiness Assessment Guide). The TRA frequently uses a maturity scale – technology readiness levels (TRLs) – that are ordered according to the characteristics of the demonstration or testing environment under which a given technology was tested at defined points in time. The TRL scale consists of nine levels, each one requiring the technology to be demonstrated in incrementally higher levels of fidelity in terms of its form, the level of integration with other parts of the system, and its operating environment than the previous, until at the final level (TRL 9) the technology is described in terms of actual system performance in an operational environment. The primary purpose of using TRLs in the DoD is to help management in making decisions concerning the development and transitioning of technology. TRLs provide a common understanding of technology status and facilitate risk management. It is important to note that although the TRL metric is ordinal, the maturing technologies along the TRL scale is not consistent. For example, moving a technology from a TRL 3 to a TRL 4 may not require the same amount of effort as moving the same technology from a TRL 6 to a TRL 7.
Biomedical TRLs
Medical products require TRL definitions and descriptions that are appropriate and unique to the technologies upon which they are based and that account for the statues and regulations that govern their development and use. In recognition of these factors, the U.S. Army Medical Research and Materiel Command (USAMRMC) established definitions, descriptions, and processes in the context of military medical research and development and Food and Drug Administration (FDA) statutory and regulatory requirements. These are provided in Appendix E of the Department of Defense Technology Readiness Assessment (TRA) Deskbook published July 2009. Below is a simplified comparison table of generalized DoD non-medical (i.e., “line”) and biomedical descriptions for each TRL.
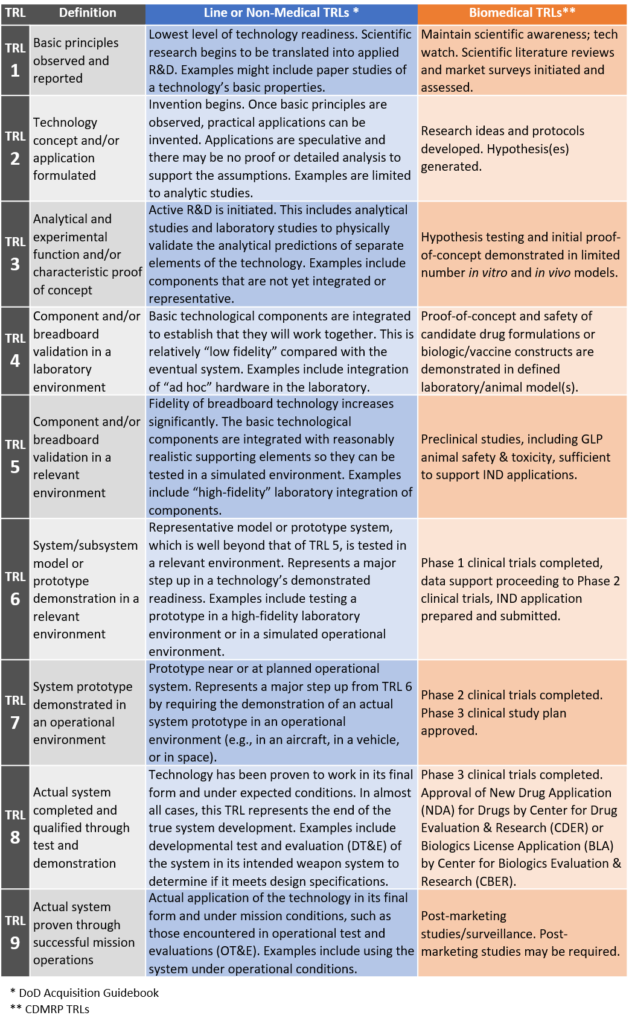
A simplified biomedical TRL example for a therapeutic candidate will progress through TRLs 1 and 2 involving basic technology research during which novel concepts are developed, hypotheses are generated, and scientific literature reviews are conducted. Further research to prove feasibility occurs during TRL 2 and 3, and these activities may include synthesizing drug candidates, identifying sites and mechanisms of action, and/or identifying device components and design. Technology development continues into TRL 4, which may involve non-GLP formulation, dose, and pharmacokinetic studies, non-GLP safety and efficacy studies, and/or identification of assays/procedures for GLP/GCP studies. Technology demonstration may continue through TRLs 5 and 6, during which applied research findings are translated into solutions for military capabilities. System/subsystem development and maturation continue through TRL 7 and TRL 8, during which Phase 1, 2, and 3 clinical trials are completed and approval is sought for New Drug Applications (NDA) or Biologics License Applications (BLA) form the Center for Drug Evaluation & Research (CDER) or the Center for Biologics Evaluation & Research (CBER), respectively. System test and operational launch occur during TRLs 8 and 9, during which the technology is deployed in actual mission environments, followed by post-marketing studies and surveillance.
In future articles, we will discuss further subcategorization of biomedical TRLs for drugs, biologics, vaccines; medical devices; and medical information management/information technology (IM/IT), as well as how these TRLs can be further translated with medical countermeasures (MCMs) that require animal use studies. In addition, various TRLs are further defined for Biomedical and MCM applications, as well as TRLs developed or categorized by Agency, and are available as resources at Tier Seven.
23 Apr
2018
