“Can my company, that is based in Canada, apply for a small business innovation research (SBIR) grant [or Department of Defense (DoD), or Congressionally Directed Medical Research Program (CDMRP), or National Institutes of Health (NIH) funding]?” For SBIR – typically no, as SBIR funds go only to small, independent U.S. businesses based on their ownership and control. For the DoD and CDMRP – typically yes, as long as J-1 Visa requirements are met and your country does not meet the criteria for designation as a State Sponsor of Terrorism. For NIH – typically yes (unless specifically excluded in the program announcement), but it also depends on the international composition of the company (e.g., domestic institution with a foreign component) and the type of grant. There are some general guidelines provided in this article, but it is very important to carefully read the Application Instructions for each funding opportunity in order to verify your company’s eligibility. The authorizing legislation and agency policies will determine whether an international or foreign individual or foreign individual or organization may apply for the grant. Double check that you are eligible in the Application Instructions, otherwise your company could expend valuable resources completing the application process for a grant or contract that your organization cannot legally receive, regardless of the merit of your application. It is also important to understand the difference between a grant or contract when seeking R&D funding from the NIH (typically grants), DoD (typically contracts) or the CDMRP. University of Pittsburgh provides a summary on the different funding mechanisms here.
As our friends from the North in Canada and all over the world provide great technology, foreign eligibility has become a more frequently asked question. As globalization continues its rapid place, deciphering a company’s components covering many countries will challenge the current eligibility determination processes. As companies from countries all over the world ask about their eligibility to seek non-dilutive funding sources that augment their private fund raising efforts, it is important that we encourage whenever possible and try to guide them to correct answers. Please let us know if there are other agencies that we can discuss and please provide additional eligibility comments or edits based on your research experiences.
Foreign vs. International Organizations
Although the terms are sometimes used interchangeably, there is an important distinction between foreign organizations and international organizations when it comes to applying for government funding. According to the NIH:
A Foreign Organization is an entity that is:
- A public or private organization located in a country other than the U.S. and its territories that is subject to the laws of the country in which it is located, irrespective of the citizenship of project staff or place of performance;
- A private nongovernmental organization (NGO) located in a country other than the U.S. that solicits and receives cash contributions from the general public;
- A charitable organization located in a country other than the U.S. that is nonprofit and tax exempt under the laws of its country of domicile and operation, and is not a university, college, accredited degree granting institution of education, private foundation, hospital, organization engaged exclusively in research or scientific activities, church, synagogue, mosque or other similar entities organized primarily for religious purposes; or
- An organization located in a country other than the U.S. not recognized as a Foreign Public Entity.
An International Organization is an organization that identified itself as international or intergovernmental and has membership from, and represents the interests of, more than one country, without regard to whether the headquarters of the organization and location of the activity are inside or outside of the U.S.
Note that foreign organizations are eligible to apply for many international programs within the NIH and DoD.
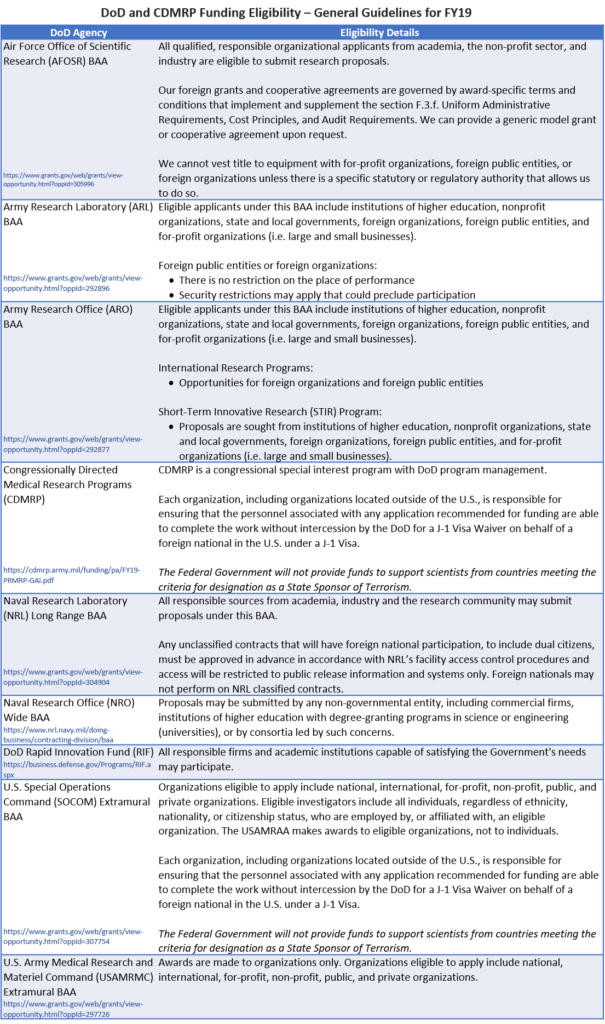
Foreign Organization Eligibility for DoD Funding Opportunities
There is no central guidance available for eligibility requirements for DoD funding programs. However, below are some of the observed most recent (i.e., FY19) eligibility details for several annually recurring broad agency announcements (BAAs) for components of the Air Force, Army, Navy, and other DoD-related agencies. It is still important to verify your eligibility within any specific funding opportunity of interest.
Foreign Organization Eligibility for NIH Funding Opportunities
The NIH provides information for foreign applicants and grantees. Section III.1.A. of each Funding Opportunity Announcement (FOA) from the NIH describes the type of institutions/organizations that are eligible to apply and Section III.1.B. provides information on the type of individuals that are eligible to apply. In addition, Chapter 16 of the NIH Grants Policy Statement describes general eligibility requirements for foreign organizations:
- In general, foreign organizations and international organizations, including public or private non-profit or for-profit organizations, are eligible to apply for research project grants, but are not eligible to submit a modular grant application. International organizations are treated as foreign organizations for the purpose of eligibility. If the FOA allows foreign organizations to apply, international organizations may apply.
- Foreign and international organizations are not eligible to apply for SBIR/STTR grants, Kirschtein-NRSA institutional research training grants, program project grants, center grants, resource grants, or construction grants.
- However, some activity codes, such as program project grants (P01), may support projects awarded to a domestic institution with a foreign component.
A foreign component is defined as performance of any significant element or segment of the project outside of the U.S. either by the recipient or by a researcher employed by a foreign organization, whether or not grant funds are expended. Activities that would meet this definition include the following:
- The involvement of human subjects or vertebrate animals at a foreign site.
- Extensive foreign travel by recipient project staff for the purpose of data collection, surveying, sampling, and similar activities.
- Any activity of the recipient that may have an impact on U.S. foreign policy through involvement in the affairs or environment of a foreign country.
Examples of other grant-related activities that may be significant are:
- Collaborations with investigators at a foreign site anticipated to result in co-authorship;
- Use of facilities or instrumentation at a foreign site; or
- Receipt of financial support or resources from a foreign entity.
- Foreign travel exclusively for consultation is NOT considered a foreign component.
Foreign institutions may apply for direct funding under selected grant mechanisms and initiatives at NIH. Unless specifically excluded by Program Announcements (PAs) or Requests for Applications (RFAs), foreign institutions are eligible to apply for most types of investigator-initiated research project grants (R03, R21, and R01 mechanisms). Foreign institutions may also receive support through subcontracts or consortium agreements, as collaborators of investigators at U.S. institutions.
Applicants from foreign institutions and international organizations must fulfill some additional requirements to register for electronic submission of grant applications. Registration can be a lengthy process and foreign organizations are especially encouraged to register early.
The NIH grant application and review processes are complex. It is also highly competitive. The likelihood of success in this process is highly dependent on experience. For these reasons, many foreign investigators choose to work in a collaborative partnership with a U.S. investigator who has a successful record of obtaining NIH funding and who can provide sound advice regarding the proposed research plan and grant application. The following are assessed as part of the review process and award decisions for applications from foreign institutions:
- Whether the project presents special opportunities for furthering research programs using unusual talent, resources, populations, or environmental conditions in other countries that are not readily available in the U.S. or that augment existing U.S. resources.
- Whether the proposed project has specific relevance to the mission and objectives of the NIH Institute or Center and has the potential for significantly advancing the health sciences in the U.S.
- Whether comparable work is being done in the U.S.
Among the institutes at NIH, the National Institute of Allergy and Infectious Diseases (NIAID) is a leader in funding foreign investigators, in large part because of the many infectious diseases in their research portfolio impact public health abroad.
Although NIAID often addresses principal investigators (PIs) as grantees, your organization submits the grant application and is therefore technically the recipient of funding. As such, when considering your eligibility for funding, it is your institution that matters most. In addition to NIAID, many of NIH’s 27 Institutes and Centers have international programs/collaborations.
Foreign Investigators
It is not required that you have U.S. affiliation or citizenship to become a PI for most NIH grants. Exceptions to this include fellowships, career development awards (with one minor exception), and training grants, for which you must be a U.S. citizen or a permanent resident (have an Alien Registration Receipt Card). PIs of small business awards are not required to have U.S. citizenship, but they must legally reside in the U.S.
Foreign investigators working on NIH-funded grants have the following requirements:
- If you are a non-U.S. citizen working at a U.S. institution, you will need to remain there long enough to finish your project.
- If you do not have a permanent visa, state in your application that your visa will allow you to remain in the U.S. long enough for you to be productive on the project.
- Your institution ensures that you have an appropriate work visa.
- Foreign investigators funded to perform select agents and toxins research must follow special procedures.
- Learn more at Research Using Select Agents.
- By U.S. law, persons from countries listed as State Sponsors of Terrorism (currently Iran, North Korea, Sudan, and Syria) may not work with any select agent.
Training opportunities for foreign national scientists are available through the NIH Visiting Program. There are two categories of program participants: Visiting Fellows, who receive awards for research training, and Visiting Scientists, who receive appointments to conduct research. NIH’s Fogarty International Center (FIC) also promotes and supports scientific research and training for foreign national scientists internationally to reduce disparities in global health. Foreign national scientists can learn about non-NIH grant and fellowship opportunities for which they may be eligible at The Directory of International Grants and Fellowships in the Health Sciences.

SBIR/STTR Programs – Majority U.S. Ownership
The Small Business Innovation Research (SBIR) program is a U.S. Government program, coordinated by the Small Business Administration (SBA), in which all federal agencies with extramural research budgets in excess of $100 million have a percentage reserved for contracts or grants to small businesses. The Small Business Technology Transfer (STTR) Program is similar in structure to SBIR and funds cooperative research and development projects with small businesses in partnership with not-for profit research institutions (such as universities) to move research to the marketplace. All federal agencies with extramural research budgets in excess of $1 billion participate in the STTR program. For SBIR projects, the small business’ principal place of business must be in the U.S. For STTR projects, the small business must be in partnership with a U.S. research institution. Agencies participating in the DoD SBIR/STTR program include the Army, Navy, Air Force, Missile Defense Agency (MDA), Defense Advanced Research Projects Agency (DARPA), Defense Threat Reduction Agency (DTRA), Defense Health Program (DHP), Special Operations Command (SOCOM), Chemical and Biological Defense (CBD), Defense Logistics Agency (DLA), Defense Microelectronics Agency (DMEA), and National Geospatial Intelligence Agency (NGA).
The SBIR/STTR program eligibility requirements are in place to ensure that the funds go only to small, independent U.S. businesses. The ownership requirement limits the program to independent firms controlled by U.S. citizens or lawful permanent residents as a way of maximizing the likelihood that the funding will stimulate innovative activity within the U.S. economy. A majority (more than 50%) of your firm’s equity (e.g., stock) must be directly controlled by one of the following:
- One or more individuals who are citizens or lawful permanent residents of the U.S.;
- Other for-profit small business concerns (each of which is directly owned and controlled by individuals who are citizens or lawful permanent residents of the U.S.);
- A combination of (1) and (2) above;
- Multiple venture capital operating companies, hedge funds, private equity firms, or any combination of these, so long as no one such firm owns or controls more than 50% of the equity. This option is allowed only for SBIR awards from agencies that are using the authority provided in § 5107 of the SBIR/STTR Reauthorization Act (majority-VC-owned authority), 15 U.S.C. § 638(dd)(1). The venture capital operating company, hedge fund or private equity firm must have a place of business located in the U.S. and be created or organized in the U.S., or under the law of the U.S. or of any State.
Note: If an Employee Stock Ownership Plan owns all or part of the concern, each stock trustee and plan member is considered an owner. If a trust owns all or part of the concern, each trustee and trust beneficiary is considered an owner.
How is ownership determined? Ownership refers to direct ownership of stock or equity of the small business. When determining a small business’ ownership, control and affiliation for the SBIR/STTR programs, SBA reviews equity ownership on a fully diluted basis. This means that SBA considers the total number of shares or equity that would be outstanding if all possible sources of conversion were exercised, including, but not limited to: outstanding common stock or equity, outstanding preferred stock (on a converted-to-common basis) or equity, outstanding warrants (on an as-exercised and converted-to-common basis), outstanding options and options reserved for future grants, and any other convertible securities on an as-converted-to-common basis.
Registering with Grants.gov as a Foreign Entity
To apply as a foreign entity for a federal grant or contract through Grants.gov, you must first ensure that you have two key pieces of information: a DUNS number and an NCAGE Code.
- A DUNS (Data Universal Numbering System) number is unique to your organization and free to acquire. Click here to begin the process.
- The NCAGE (NATO Commercial and Government Entity) Code is required for all foreign entities seeking a federal grant. Click here to apply for an NCAGE Code.
After you have both the DUNS number and NCAGE Code, there is one final step before registering with Grants.gov: You need to register with the System for Award Management (SAM). See the registration guide for international entities form SAM.gov. Once you have registered with SAM, you are ready to register with Grants.gov.
Additional Options for Small Organizations
As described in the Grants.gov Exploring Eligibility blog series, it is common for small in-country foreign organizations to partner with larger organizations receiving grant funding from the U.S. government. In such cases, specific requirements for applying may vary depending on the grant-making agency. For example, the NIH explains that grants awarded to a consortium of NGOs may be passed through a single applicant entity (i.e., direct and primary recipient of funds). In this case, one organization will manage the application and partner organizations will be required to abide by the rules and guidelines outlined by the grant-making agency. Alternatively, U.S. missions will sometimes post funding opportunities for in-country foreign organizations whose work aligns with a programmatic objective.
As mentioned above, if there are other agencies that we can discuss and or if you can provide additional eligibility information or edits based on your research experiences, please comment below or contact us.
13 Feb
2019