
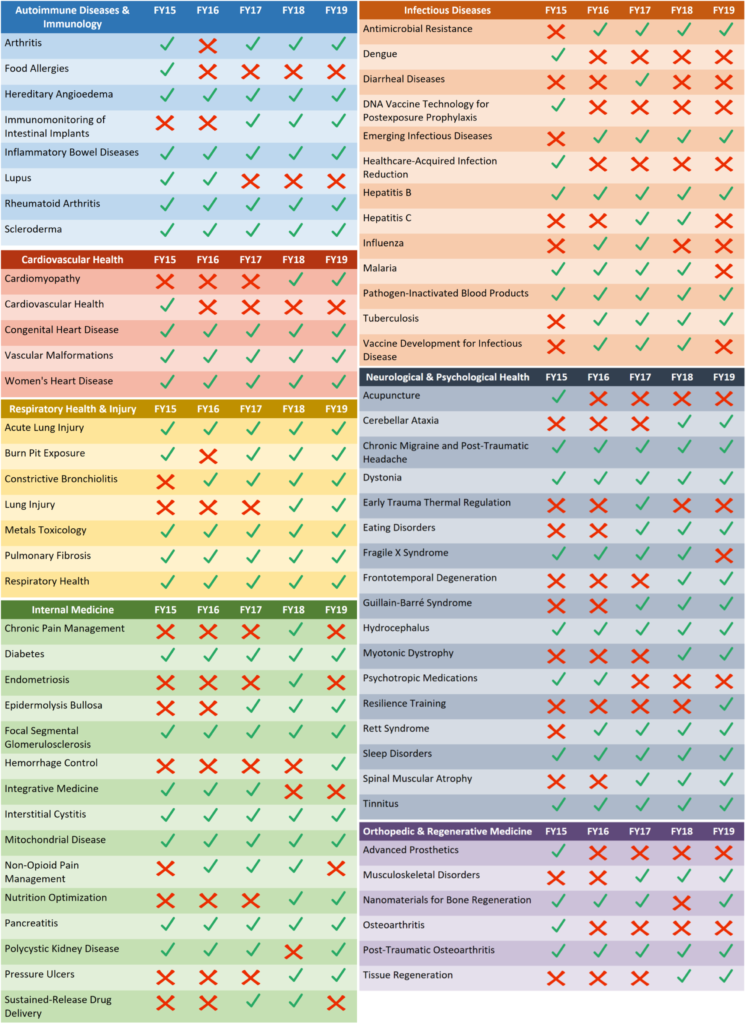
A great source of non-dilutive funding for small business concerns (SBCs) and Universities is the Congressionally Directed Medical Research Program (CDMRP summary). CDMRP currently has 32 research programs, which includes the Peer Reviewed Medical Research Program (PRMRP). There are also 50 topic areas in the PRMRP that your company can apply for in FY19. These topics are selected and funded by Congress. These topic areas can vary year-by-year (see the graphic below on a 5-year history of the PRMRP topic areas), so it is important to monitor the topic areas on an annual basis.
CDMRP was established in 1999 with the goal of improving the health and well-being of military Service members, Veterans, and their families. CDMRP is unique in that the $1.02B FY19 program was funded by Congressional Special Interest Research, Development, Testing & Evaluation (RDT&E) and managed by the Department of Defense (DoD). CDMRP is programmatically reviewed by the DoD, Department of Veterans Affairs (VA), and the Office of the Assistant Secretary for Defense Health Affairs (OSD DHA) and then approved by the DoD and Defense Health Agency (DHA) Research and Development Directorate. Under U.S. Army Medical Research and Materiel Command (USAMRMC), the CDMRP provides Defense Health Program (DHP) RDT&E program execution management support for six core research program areas, each managed by a Joint Program Committee (JPC). The CDMRP, in partnership with the JPCs, supports development of program announcements, solicitation and review of applications, full life-cycle management of awards, as well as program evaluation and planning. This is accomplished by identifying and funding military health-related research of exceptional scientific merit. The PRMRP is committed to funding basic, translational, and clinical research that will strongly impact the understanding of disease and injury etiology, as well as the development and implementation of devices, therapies, and clinical guidance that change prevention, diagnosis, and treatment for numerous conditions.
Review Process
The USAMRMC adheres to a two-tier review system for each of the CDMRP programs: Scientific Peer Review (Tier 1) and Programmatic Review (Tier 2).
Peer Review
Each peer review panel includes a Chair, scientific reviewers, and a non-voting Scientific Review Officer. Applications are discussed individually and assessed using the evaluation criteria published in the program announcement. The Scientific Review Officer is responsible for preparing a Summary Statement that includes the applicants’ abstracts, evaluation criteria, overall scores, peer reviewers’ written comments, and the essence of panel discussions. The Summary Statement is used to report peer review results to the Programmatic Panel.
Programmatic Review
The Programmatic Panel includes representatives of each branch of the military Services, the VA, the OSD DHA, and ad hoc reviewers. Programmatic review is a comparison-based process that considers scientific evaluations across all disciplines and specialty areas. Applications that were highly recommended in the technical merit review process are not automatically recommended for funding. Programmatic review criteria are ratings and evaluations of the scientific review panels, programmatic relevance, adherence to the intent of the award mechanism, military relevance, program portfolio composition, and relative impact. Following programmatic review, the Commanding General, USAMRMC, and the Director of the DHA Research and Development Directorate approve funding for the recommended applications.
Consumer Involvement
The CDMRP has a third line of review for applications that is unique and important. A required document for each application is a “Lay Abstract” for the Consumer. This “personal perspective, passion, and a sense of urgency” is critical to accelerating CDMRP-funded technologies. This “Consumer Involvement” description is from the CDMRP website: “CDMRP welcomes patients, survivors, family members, and advocates to play a pivotal role in the future of biomedical research funding. To transform healthcare for our Service members and the American public, the CDMRP looks to those who have the most experience, who understand the effects of a disease, an injury, or a condition – the individuals (consumers) living with breast cancer, orthopaedic injury, Parkinson’s disease, etc. By integrating patients, survivors, family members and/or care takers into the scientific review process, the CDMRP is able to enrich the scientific review with personal perspective, passion, and a sense of urgency that ensures the human dimension is incorporated in the research focus. Over 2,000 consumers have served as Peer and Programmatic reviewers since 1995.” A wonderful collection of videos and narratives on “Consumer Experiences from 2005-2018 can be found here.
Timeline from Submission to Award
More often than not, pre-applications are a required part of the two-step submission process for PRMRP and other CDMRP awards; however, a few require only a short letter of intent (LOI) for a “pre-app.” In the case where a pre-proposal is required (not just an LOI), the application process typically follows the general timeline:*
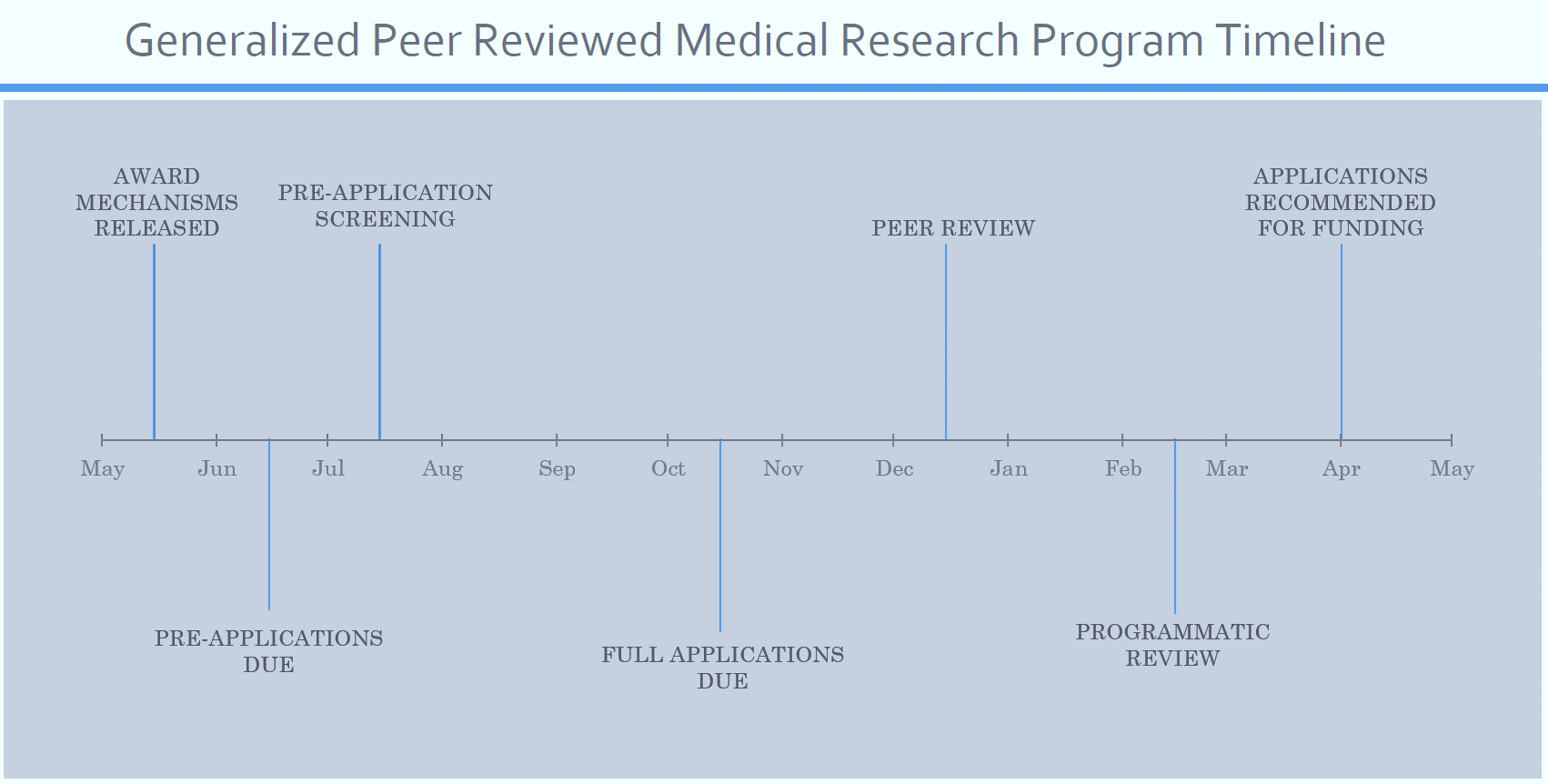
**FY19 PRMRP Funding Opportunities were released unusually early (January 15, 2019). This year pre-applications are due in March, most full applications are due in July, peer review will be in August, and programmatic review will be in October/November 2019
Occasionally, when pre-applications are not required, the timeline may be abbreviated and moved forward by a few months, with full applications due around July and the review process (peer and programmatic) occurring from approximately September to December.
PRMRP Award Mechanisms
Clinical Trial Award (CTA)
The Clinical Trial Award supports the rapid implementation of clinical trials of novel interventions with the potential to have a significant impact on a disease or condition addressed in the topic area(s) of interest. Projects may range from small proof-of-concept trials to large-scale, definitive trials. Principal Investigators (PIs) must be at the Assistant Professor level or above (or equivalent) to be eligible to submit for the CTA.
In FY16, the PRMRP CTA announcement received 171 pre-applications, of which 74 were invited to submit full applications. Of the 64 full applications received, 11 (17.2%) were recommended for a total of $82.3M in funding. For FY19, the CDMRP expects to allot approximately $58.5M to fund approximately 9 CTA applications.
The FY19 CTA program announcement has been released, with pre-proposals due by March 14, 2019. There is no maximum funding limit (requested funding must be justifiable and appropriate for the scope of work proposed), and the maximum period of performance is four years.
Discovery Award (DA)
The Discovery Award supports the exploration of a highly innovative new concept or untested theory. The DA is not intended to support the logical progression of an already established line of questioning. The DA does not fund clinical trials. This is the only PRMRP award mechanism for which a pre-proposal submission is not required. PIs must be postdoctoral or clinical fellows (or equivalent) or above to be eligible for the DA.
In FY18, the PRMRP DA announcement received 523 full applications, of which 77 (15%) were recommended for a total of $23.1M in funding. For FY19, CDMRP expects to allot approximately $18M to fund approximately 60 DA applications.
The FY19 DA program announcement has already been released, with letters of intent due by March 28, 2019. The maximum funding for a DA is $200,000 for direct costs (plus indirect costs), for a maximum period of performance of two years.
Focused Program Award (FPA)
The Focused Program Award supports a synergistic, multidisciplinary research program of at least four distinct but complementary projects addressing an overarching goal. Projects should work together to answer critical questions, resolve differing hypotheses, and translate laboratory findings to clinical applications. FPA projects may range from exploratory/ hypothesis-developing to small-scale clinical trials that together will address the overarching goal. Lead PIs must be at the Full Professor level or above (or equivalent) to be eligible for the FPA. Project leads must be at the Assistant Professor level or above (or equivalent).
In FY17, the PRMRP FPA announcement received 128 pre-applications, of which 34 (27%) were invited to submit full applications. Of the 31 full applications received, 3 (9.7%) were recommended for a total of $29.6Min funding. For FY19, the CDMRP expects to allot approximately $43.2M to fund approximately 4 FPA applications.
The FY19 FPA program announcement has already been released, with pre-proposals due by March 14, 2019. The maximum funding for an FPA is $7.2 million for direct costs (plus indirect costs), for a maximum period of performance of four years.
Investigator-Initiated Research Award (IIRA)
The Investigator-Initiated Research Award supports research that will make an original and important contribution to the field of research or patient care in the topic area(s) of interest. The IIRA does not fund clinical trials. PIs must be at the Assistant Professor level or above (or equivalent) to be eligible for the IIRA.
In FY17, the PRMRP IIRA announcement received 851 pre-applications, of which 417 (49%) were invited to submit full applications. Of the 409 full applications received, 47 (11.5%) were recommended for a total of $91.4Min funding. For FY19, the CDMRP expects to allot approximately $79.6M to fund approximately 42 IIRA applications.
The FY19 IIRA program announcement has already been released, with pre-proposals due by March 14, 2019. The maximum funding for an IIRA is $1.2 million (or $1.5 million for applications including a Partnering PI Option) for direct costs (plus indirect costs), for a maximum period of performance of three years.
Technology/Therapeutic Development Award (TTDA)
The Technology/Therapeutic Development Award supports the translation of promising preclinical findings into clinical applications for prevention, detection, diagnosis, treatment, or quality of life. The TTDA is product-oriented (e.g., device, drug, clinical guidelines). The product(s) to be developed may be a tangible item such as a pharmacologic agent (drugs or biologics) or device, or a knowledge-based product. However, the TTDA does not fund clinical trials. PIs must be at the Assistant Professor level or above (or equivalent) to be eligible for the TTDA.
In FY17, the PRMRP TTDA announcement received 302 pre-applications, of which 129 (43%) were invited to submit full applications. Of the 122 full applications received, 20 (16.4%) were recommended for a total of$73.1M in funding. For FY19, the CDMRP expects to allot approximately $72M to fund approximately 16 TTDA applications.
The FY19 TTDA program announcement has already been released, with pre-proposals due by March 14, 2019. The maximum funding for a TTDA is $3 million for direct costs (plus indirect costs), for a maximum period of performance of three years.
PRMRP Topic Areas
The PRMRP supports research in Congressionally specific topic areas that span across a wide range of health conditions, diseases, and research fields for the benefit of military Service members, Veterans, military health system beneficiaries, and the American public.
Annual Changes in Research Topics
Applications submitted to the PRMRP must address at least one of the Congressionally Directed Topic Areas. Topic Areas are determined on an annual basis.
What Now?
If your SBC has dual-use biomedical or human system technologies that can improve the operational readiness, situational awareness, or healthcare for our military personnel, Veterans, and their families, consider utilizing DoD non-dilutive funding resources such as the PRMRP to accelerate your technologies to DoD, VA, civilian, and international marketplaces. Contact us to learn how Tier Seven can assist your company in connecting with potential DoD researchers and forming research collaborations.
06 Mar
2019
