
What is MTEC?
The Medical Technology Enterprise Consortium (MTEC) was established as an enterprise partnership to facilitate research in and translation of life science technologies. MTEC was formed in response to the U.S. Government’s appeal to speed delivery of medical technology that protects, treats, and optimizes the health and performance of U.S. military personnel. The goal of this Consortium is to “conduct research and development activities that advance the state-of-the-art as well as medical science and technology skills in the fields that are needed to develop and transition new and improved medical technologies to support current and future U.S. military personnel health needs.” In other words, there are very challenging capability gaps present in our biomedical and human weapon system technologies arsenal. Building these complex capabilities will require a focused and well managed public-private hybrid effort (e.g., DoD, other Federal Agencies and multiple private industry companies) to provide solutions. For example, one of the first projects was a “Brain Machine Interface Prototype Development for Vision Restoration.” This effort requires subject matter expertise and state-of-the-art technologies from many entities to include: vision, retinal prostheses, microelectronics and microchip electrodes, microelectrodes, neurosurgery/deep brain stimulation and many others. This effort also requires extensive collaboration and cross disciplinary innovations that are typically not possible for one entity to provide. These Consortiums must be assembled and managed so that all entities can benefit from the collaboration, and most importantly, that our warfighters, Veterans and their families will benefit.
Why Build MTEC?
Commercial investments now accelerate most medical technology development. These dual-use (military and civilian) technologies are of significant interest to the DoD, but the high barriers to entry, including the long timelines inherent to the federal acquisition system, can position the DoD as a challenging market to enter for companies that are focused on civilian commercial markets. The DoD has responded to provide flexibility and promote innovation and participation by both traditional and non-traditional defense contractors in cost shared public-private partnerships. MTEC is an agreement instrument to remove many of these development, transition and commercialization challenges.
MTEC is a nonprofit corporation collaborating with multiple government agencies. MTEC operates under a 10-year renewable Section 845 Prototype Other Transaction Agreement (OTA) with the U.S. Army Medical Research and Materiel Command (USAMRMC). Other Transaction (OT) is a special funding vehicle used by Federal Agencies for obtaining or advancing R&D or prototypes. OT are not contracts, grants, or assistance agreements, and are not subject to Federal Acquisition Regulations (FAR) and DoD Grant and Agreement Regulations (DoDGAR) acquisition policies and procedures. OTs are an instrument, unlike contracts, that are implemented to facilitate mutually beneficial R&D collaborations between industry, academia and the Government.
How Does MTEC Function?
The Advanced Technology Institute in South Carolina received the award from USAMRMC in August 2015 to organize MTEC. Since then, MTEC has established corporate governance, established business procedures and systems, and secured tax exempt status from the IRS. The first solicitation was issued in March 2016.
The objectives of MTEC are:
- 1. Biomedical research and prototyping;
- 2. Exploration of private sector technology opportunities;
- 3. Technology transfer; and
- 4. Deployment of intellectual property and follow-on production.
Technology Areas
MTEC’s technology focus areas are listed below and described in further detail here.
- Prevention, Diagnosis and Treatment of Infectious Diseases – Discover disease causing microorganisms and develop vaccines/drugs to prevent and treat infectious diseases rapidly.
- Care of Combat Casualties – Reduce killed-in-action rate of warfighters, reduce the morbidity of combat injuries, and reduce the medical footprint on the battlefield by providing biologics, pharmaceuticals, and devices that enhance the capability of the medical staff to effectively treat casualties as close to the location and time of injury as possible.
- Clinical and Rehabilitative Medicine – Develop technologies and products to replace or regenerate human cells, tissues, or organs to restore or establish normal functions such as tissue regeneration, bone scaffolding, and stem cell enabled treatments to severely injured Service members.
- Military Operational Medicine – Develop effective countermeasures against stressors and maximize health, performance, and fitness. Includes injury prevention and reduction, psychological health and resilience, and environmental health and protection.
- Medical Training and Health Information Science – Develop products and processes that increase patient safety and quality of care through simulation-based technologies and health informatics systems to include the development of products and processes that implement or improve medical simulation and training, health informatics and mobile health, and decision support tools and physiological models.
- Advanced Medical Technologies – Develop initiatives and products that will increase medical mobility while ensuring access to essential medical expertise and support regardless of the operating environment. Includes e-health, digital warrior, hospital of the future integrative medicine, advanced orthopedic devices and treatments, advanced medical imaging technologies, robotic technologies to treat and rescue battlefield casualties, nanotechnology and biomaterials for diagnosis and therapy, technologies for treating neurological injuries, and regenerative medicine.
Solicitations
MTEC is only in its second year of operation. As such, there have only been solicitations for 2016 and 2017. Typically, MTEC releases two solicitations per year, but this appears to be increasing. Solicitations tend to be open for a short period of time (4 to 6 weeks), so it is necessary to be prepared and act quickly once they are released. Upcoming solicitations can be viewed here.
2016 MTEC Solicitations
- Brain Machine Interface Prototype Development for Vision Restoration
- Horus Vision Restoration Project
- Regenerative Medicine
MTEC Projects Awarded
- Commercial Scale Up of Bone Marrow-Derived Mesenchymal Stem Cells for Regenerative Medicine (BioBridge)
- Manufacture of a Settable Nanocrystalline Hydroxyapatite/Polymer Composite Bone Graft (Vanderbilt)
- Development of Universal Media for the Support and Expansion of Human Cells for Regenerative Medicine Manufacturing (RegenMed)
- Development of a Universal Bioink with Tunable Mechanical Properties for Regenerative Medicine Additive Manufacturing of Clinical Products (RegenMed)
2017 MTEC Active and Upcoming Solicitations:
(Requests for Project Information are also listed for other topics)
- Dengue Human Infection Model (DHIM) Prototype Development
- Extracorporeal Life Support (ECLS)
- Operational Architectures to Support Military Medical Training Simulations
- Permanent Vascular Repair (PVR)
- Prototype Acceleration for 1) Wound care/anti-infectives to include point of injury wound care; and 2) Regenerative medicine.
The MTEC Proposal-Award Cycle is illustrated below. Solicitations may or may not require submission of white papers prior to full proposal submissions. If a full proposal is recommended for award but adequate funding is not available from the original sponsor at the time of source selection, the sponsor may then decide to place that proposal in the “Basket.” Basket proposals may receive funding up to two (2) years after the original Request for Project Proposal (RFP) due date from either the same sponsor, a different federal sponsor, or a private sector sponsor.
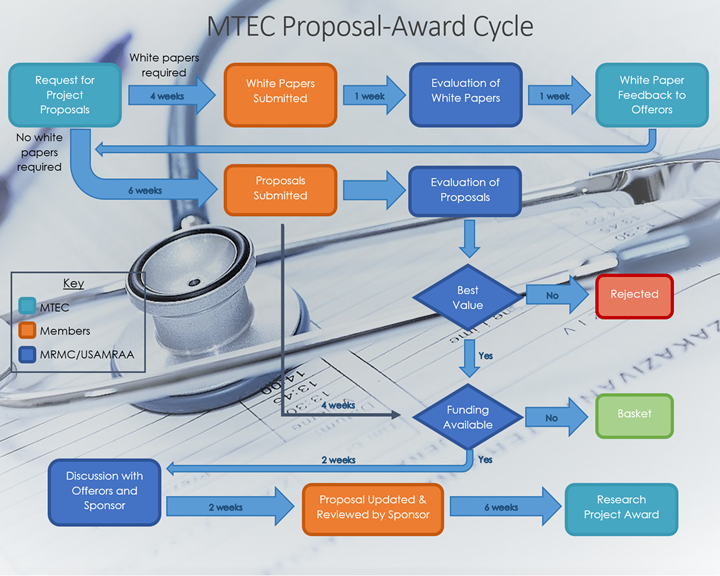
Adapted from MTEC Industry Day Presentation, March 1, 2016 and How to Work with MTEC Webinar, November 22, 2016
It is important to note that all applicants to MTEC RFPs must be MTEC Members [MTEC Consortium Member Agreement]. Similarly, the Proposal Preparation Guide and other submission resources are only accessible through the “Members Only” portion of the MTEC website. So, what does membership entail?
Membership
As of June 2017, the Consortium consisted of 108 members. Consortium Members may include any of the following:
- Small and Large Businesses
- Contract Research Organizations
- “Non-Traditional” Government Contractors
- Not-For-Profit Organizations
- Academic Research Institutions
The process to become a MTEC member is relatively simple; it requires completing the Consortium Membership Agreement (CMA), which includes the Membership Application and Proprietary Information Exchange Agreement. Membership fees are charged annually on October 1st at $500 for multi-member organizations, $5,000 for large businesses, and $1,000 for others (e.g., small businesses, academic research institutions, not-for-profits). The MTEC 2nd Annual Membership Meeting was held at Southwest Research Institute in San Antonio, Texas, on Thursday, March 30th, 2017. A networking event with poster displays by current members and invited government sponsors kicked-off the meeting on Wednesday, March 29th, 2017.
Additional Resources
What Now?
Tier Seven would like to hear your comments about and experiences with MTEC. Tier Seven is building a consortium in a topic-focused area. Agreement mechanisms like the OTs utilized by MTEC and other DoD organizations could provide some great opportunities to find solutions for existing capability gaps. We appreciate the hard work that MRMC put into creating MTEC to assist our warfighters, Veterans and their families. Tier Seven can assist your company in connecting with potential DoD partners and forming research collaborations.
03 Apr
2017
