
Traumatic Brain Injury (TBI) is a significant health issue that occurs at a high rate in military populations. According to the Defense and Veterans Brain Injury Center, more than 380,000 Service members have been diagnosed with TBI since 2000. TBI is also a significant public health burden that most commonly is a result of car accidents, falls, or sports injuries [CDC]. The Department of defense (DoD) and Veterans Affairs (VA) have focused many research efforts (e.g., CARE Consortium) on improving head injury prevention, diagnostic capabilities (e.g., neuroimaging, smartphone devices), and therapeutic interventions for TBI and its sequelae (e.g., post-concussive symptoms, neurpsychiatric conditions). Many of these research programs are DoD funded, and a significant amount of TBI R&D funding is provided by the Congressionally Directed Medical Research Programs (CDMRP), which is a great source of non-dilutive funding or capital (NDC) for small businesses working on biomedical technologies.
Operational support for the U.S. Army’s Joint Program Committees (JPCs) is provided by multiple execution agents, including the Congressionally Directed Medical Research Programs (CDMRP), which is a Congressional special interest funded program that is managed by the DoD. CDMRP provides program and award management support primarily for basic through translational research (e.g., clinical trials) and also works closely with the JPCs to transition products to advanced development. Two of these Congressionally funded and DoD managed programs are the Psychological Health/Traumatic Brain Injury (PH/TBI) Research Program and the Defense Medical Research and Development Program (DMRDP).
There are several other CDMRP research programs with an emphasis on TBI research topics. These include the Epilepsy Research Program (ERP), the Peer Reviewed Alzheimer’s Research Program (PRARP), and the Alcohol and Substance Abuse Disorders Research Program (ASADRP).
Psychological Health/Traumatic Brain Injury (PH/TBI) Research Program
The Psychological Health/Traumatic Brain Injury (PH/TBI) Research Program of the CDMRP aims to establish, fund, and integrate both individual and multi-agency research efforts that will lead to improved prevention, detection, and treatment of the effects of traumatic stress and traumatic brain injury on function, wellness, and overall quality of life for Service members as well as their caregivers and families. PH/TBI program announcements are released multiple times each year.
Focus Areas
The PH/TBI Research Program focuses on various aspects of TBI, including:
- Physics of Blast;
- Rehabilitation and Reintegration;
- Neuroprotection and Repair;
- Treatment and Clinical Management;
- Families and Caregivers; and
- Field Epidemiology.
The PH/TBI Research Program often released solicitations as part of the Military Operational Medicine Research Program (JPC-5/MOMRP), the Combat Casualty Care Research Program (JPC-6/CCCRP), and the Clinical and Rehabilitative Medicine Research Program (JPC-8/CRMRP) with focus areas in line with the missions of these research programs. The FY17 PH/TBI Focus Areas involving TBI include:
JPC-8/CRMRP
- Mechanisms: Investigate mechanisms of recovery following isolated or cumulative TBI(s) in Service member-relevant injuries. Investigators should explore at least one of the following:
- Cognitive Deficits – To inform novel rehabilitation interventions that lead to the recovery of cognitive function.
- Sensory or Sensorimotor Dysfunction – To inform novel rehabilitation interventions that lead to recovery of sensory or sensorimotor function.
- Ecological Assessment: Develop, evaluate, and/or validate return-to-duty outcome measures following rehabilitation in patients with TBI.
- Cognitive Rehabilitation: Generate new knowledge to confirm whether novel or standard-of-care rehabilitation interventions are effective in remediating cognitive impairments (e.g., memory, processing speed, executive functioning) and functional limitations after TBI by addressing one or more of the following: optimal cognitive rehabilitation prescription patterns; optimization of combination therapies to produce synergistic treatment effects across multiple domains (e.g., cognition and pain); identification of patient characteristics presumed to affect outcomes and/or effectiveness of therapies.
- Vestibular Rehabilitation and Mechanisms of Recovery: Generate new knowledge to remediate symptoms (e.g., dizziness, vertigo, motion intolerance), impairments (e.g., gaze, postural and dynamic instability), functional limitations, and barriers to participation (e.g., readiness to return-to-duty) associated with post-traumatic dizziness and/or vestibular pathology in patients with TBI.
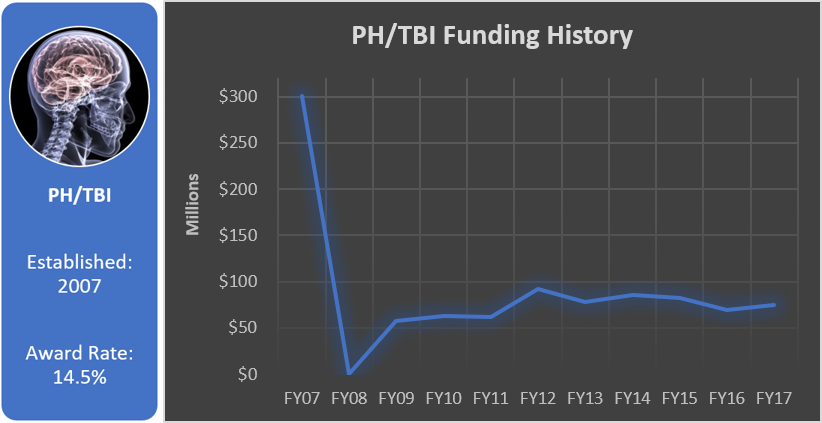
Funding History
Total appropriated funds (2007, 2009-2017): $964.7 million
Defense Medical Research and Development Program (DMRDP)
Like the PH/TBI Research Program, CDMRP provides Defense Medical Research and Development Program (DMRDP) execution management support for the JPCs/core research programs of the Defense Health Program (DHP);
- JPC-1: Medical Simulation and Information Sciences Research Program (MSISRP)
- JPC-2: Military Infectious Diseases Research Program (MIDRP)
- JPC-5: Military Operational Medicine Research Program (MOMRP)
- JPC-6: Combat Casualty Care Research Program (CCCRP)
- JPC-7: Radiation Health Effects Research Program (RHERP)
- JPC-8: Clinical and Rehabilitative Medicine Research Program (CRMRP)
Focus Areas
The DMRDP often releases solicitations as part of the JPC-5/MOMRP, JPC-6/CCCRP, and JPC-8/CRMRP with focus areas in line with the missions of these research programs. The FY17 DMRDP Focus Areas involving TBI include:
JPC-6/CCCRP
- Improving the Characterization of TBI:
- Identification and/or characterization of TBI biomarkers that are specific to TBI alone and/or TBI with concomitant injuries (e.g., burn, hemorrhage) and identification of biomarker profile specific to TBI pathology and treatment effectiveness;
- Development of targeted therapies, devices, or clinical guidelines to improve diagnosis/ stabilization/ treatment of TBI casualties with and without concomitant polytrauma based upon the precise characterization and individualized assessment of affected domains;
- Improve TBI treatment decision capabilities based upon the precise characterization and individualized assessment of TBI pathology (e.g., contusion, diffuse axonal injury, subdural hematoma, epidural hematoma);
- Improve the understanding of risk factors and outcomes associated with specific brain pathology (e.g., contusions, diffuse axonal injury, hematomas) with the end state goal of improving prognosis and optimizing recovery; and
- Improve the understanding of the role of genetic factors and/or physiology status on short- and long-term consequences of combat-related TBI.
- Understanding the factors that influence and/or inform patient responsiveness to TBI therapeutic interventions:
- Pharmacodynamic characterization of therapeutics with respect to patients responsive to treatment;
- Targeting therapeutics to the blood-brain barrier in response to specific brain injuries (e.g., contusions, diffuse axonal injury, subdural hematoma, epidural hematoma);
- Understanding the impact of sex-associated differences on the efficacy of TBI treatments;
- Understanding the potential role of genetics and sex-associated differences in TBI treatment decisions and TBI treatment response;
- Identification of biomarkers of TBI that inform treatment effectiveness and stage of recovery; and
- Identify the impact of environmental factors (such as altitude, vibration, and/or temperature) that negatively affect TBI outcomes. Identify potential treatments and/or interventions to mitigate these.
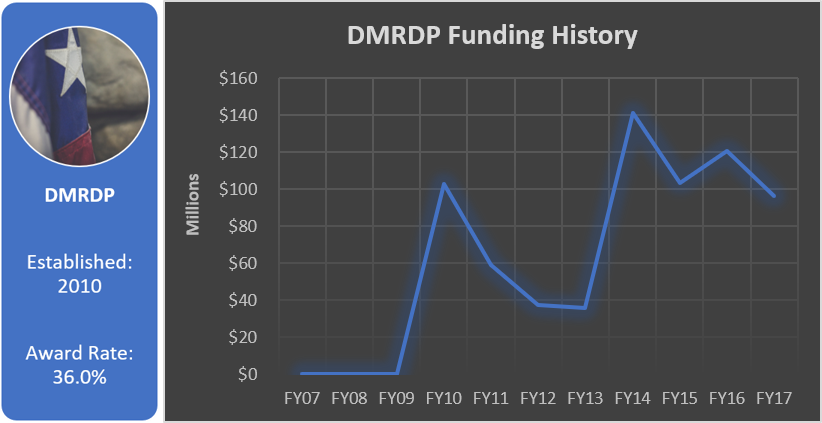
Funding History
Total appropriated funds (2010-2017): $695.6 million
Epilepsy Research Program (ERP)
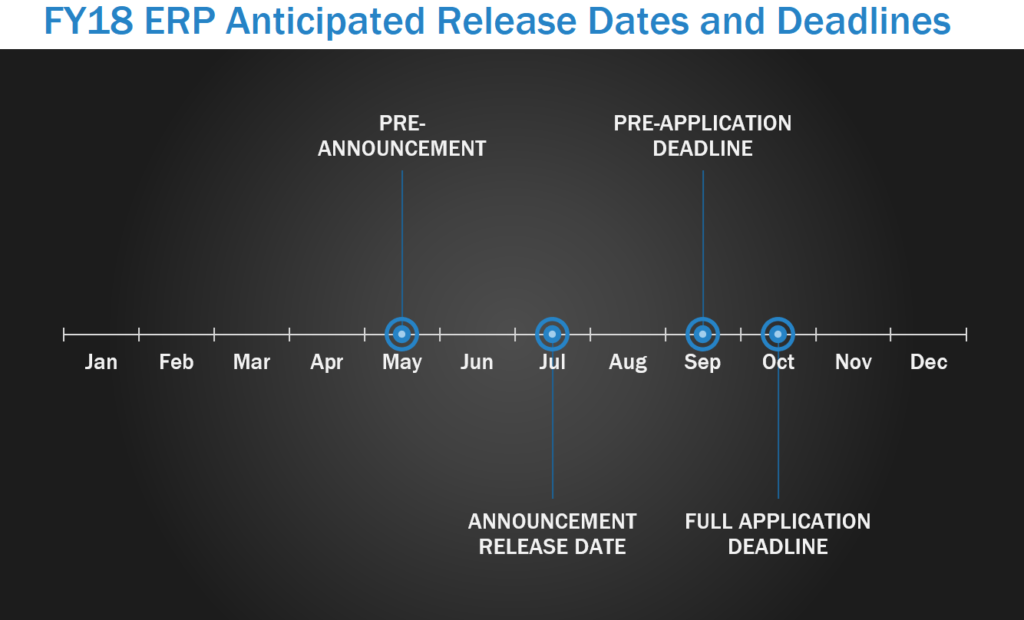
The Epilepsy Research Program (ERP) was initiated to develop an understanding of the magnitude of post-traumatic epilepsy (PTE) within the military and to expand research into the basic mechanisms by which TBI produces PTE. ERP pre-announcements are usually released in May, with program announcements released around July. In FY17, letters of intent (LOI) were due in September, with full applications due within one month of the LOI deadline.
Focus Areas
Focus Areas for the FY17 ERP include:
- Epidemiology: Epidemiological characterization of PTE following TBI, which may include:
- Risk factors such as demographics, genetic factors, organic head injury factors, or type of insult;
- Differentiation of PTE and psychogenic non-epileptic seizures (PNES);
- Outcomes including latency to epilepsy, morbidities and comorbidities, and mortality; or
- Pre-existing conditions including psychological and psychiatric risk factors.
- Markers and Mechanisms: Identifying markers or mechanisms (via clinical prospective or preclinical models) that address PTE:
- Early detection;
- Diagnosis;
- Prognosis;
- Morbidity;
- Comorbidity;
- Mortality; and/or
- Risk stratification.
- Models of PTE: Development of new models or better characterization of existing etiologically relevant models for PTE, including repetitive TBI; and
- Psychogenic Non-epileptic Seizures: Exploration of the epidemiology, mechanisms, risk factors, or markers of PNES subsequent to TBI.
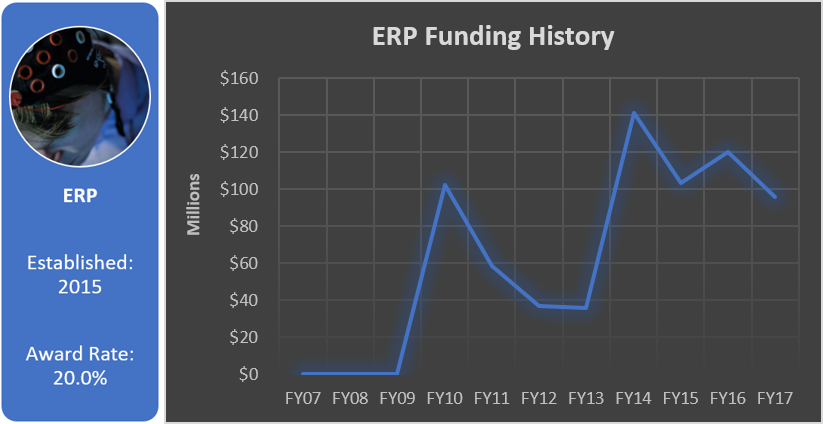
Funding History
Total appropriated funds (2015-2017): $22.5 million
Peer Reviewed Alzheimer’s Research Program (PRARP)
Military personnel and others living with TBI face an increased risk for developing Alzheimer’s-like dementia, aggression, memory loss, depression, and symptoms similar to those of other neurological diseases. The Peer Reviewed Alzheimer’s Research Program (PRARP) was initiated to address the long-term consequences of TBI as they pertain to Alzheimer’s disease (AD) and AD-related dementias (ADRD). PRARP pre-announcements are usually released in May, with program announcements released around July. In FY17, LOI were due in September, with full applications due within one month of the LOI deadline.
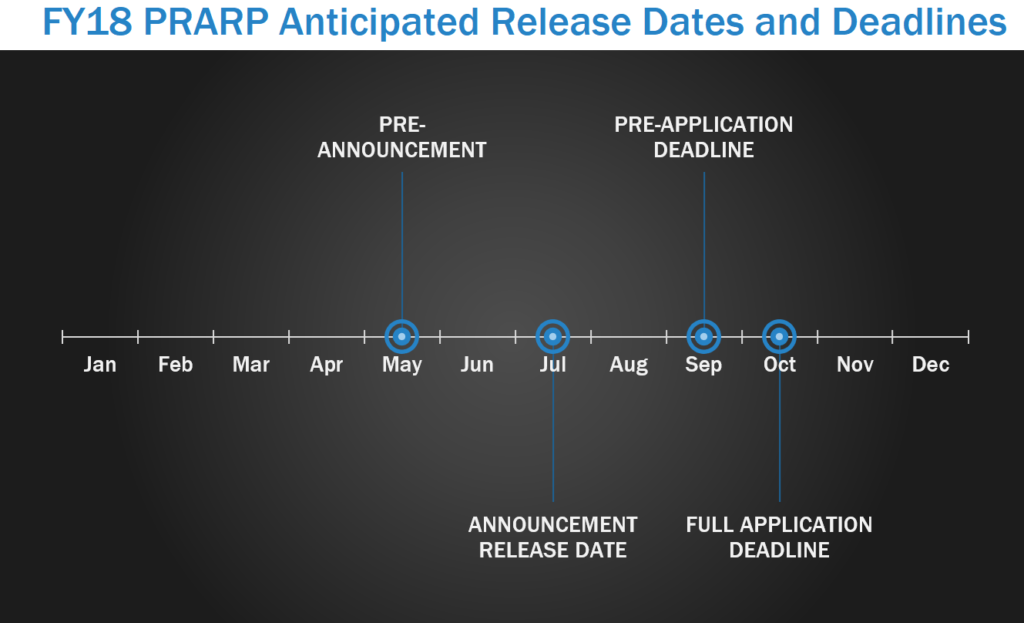
Focus Areas
The Overarching Challenges FY17 PRARP are:
- Paucity of Research Resources;
- Paucity of Clinical Studies;
- Diagnostic Technologies, Tests, Biomarkers or Devices;
- Quality of Life;
- Caregiver Burden; and
- Epidemiology.
Applications should address at least one Focus Area in support of the Overarching Challenges. The FY17 PRARP Focus Areas include:
- Genomics/Proteomics: Studies or technologies (e.g., genetic, proteomic, bioinformatics and epigenetic strategies) intended to characterize neurological change(s) associated with TBI and subsequent AD/ADRD. In addition, relevant technologies or tests may be considered under this focus area.
- Mechanisms of Pathogenesis: Identification of contributing mechanisms (e.g., pathology of Tau, non-neuronal cells, inflammatory factors, and vascular contributions) associated with TBI and subsequent AD/ADRD pathogenesis.
- Quality of Life: Research intended to alleviate, stabilize, or characterize the symptoms, or deficits, common to TBI, AD and ADRD. Examples of research in this Focus Area include: Identification and management of co-morbidities and modifiable risk factors (e.g., sleep apnea, obesity); cognitive training interventions; studies of health and wellness and behavioral interventions.
- Caregiver Support: Research intended to reduce the burden of care on the caregiver for individuals living with the common symptoms or deficits of TBI and AD/ADRD. Examples of research in this Focus Area include: Caregiver training, home-based support, behavioral interventions, and relationship interventions.
- Biomarkers: Development of strategies to diagnose, prognose, or characterize neurological changes or risk factors associated with TBI and subsequent AD/ADRD (e.g., fluid-based, imaging, physiological, and clinical approaches).
- Novel Target Identification: Basic research (non-human) directly leading to identification of new targets for the development of existing or new investigational medicines, drugs, or agents.
- Epidemiological Research: Research focusing on the incidence, distribution, and other factors relating to health of individuals affected by TBI and subsequent AD/ADRD.
Funding History
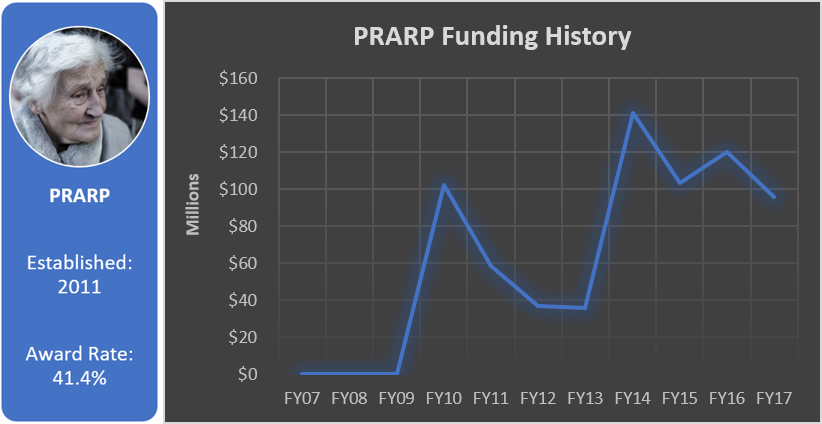
Total appropriated funds (2014-2017): $54.0 million
Alcohol and Substance Abuse Disorder Research Program (ASADRP)
The Alcohol and Substance Abuse Disorders Research Program (ASADRP) seeks research to explore integrated approaches to address alcohol and substance use disorders (ASUD), especially related to TBI and post-traumatic stress disorder (PTSD), through multidisciplinary, team-based research efforts that translate basic knowledge into enhanced clinical pharmacological treatment protocols. In FY17, the ASADRP pre-announcement was released in May, with program announcements released in June. LOIs were due in September with full applications due within one month of the LOI deadline.
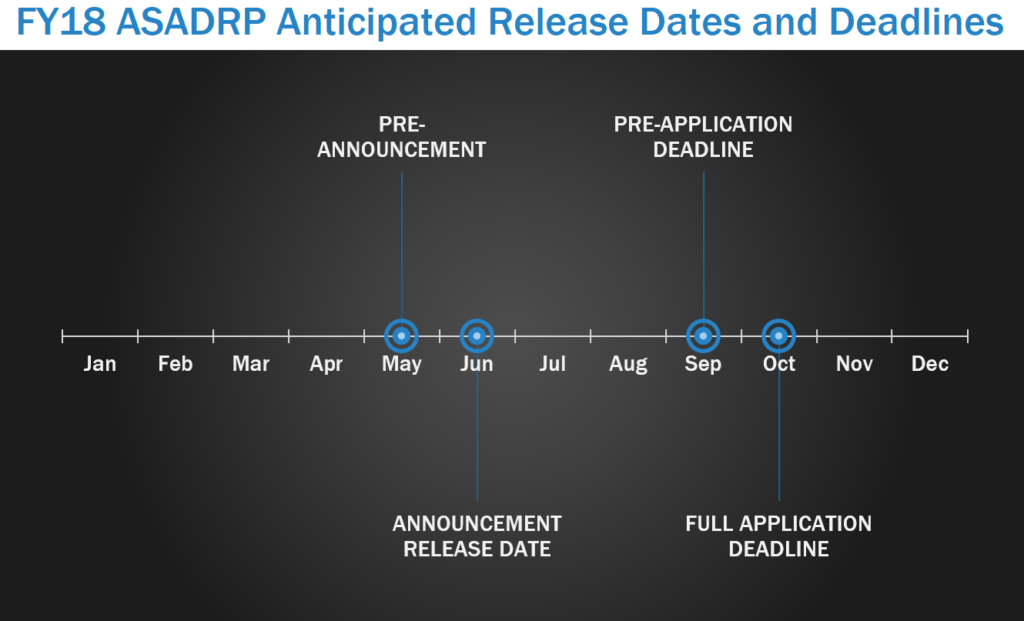
Focus Areas
The ASADRP goal for FY17 is to organize multidisciplinary, team-based translational research efforts to:
- Identify promising compounds;
- Conduct proof-of-principle basic research to determine which compounds are most appropriate for human research trials; and
- Conduct human proof-of-concept with promising compounds.
Funding History
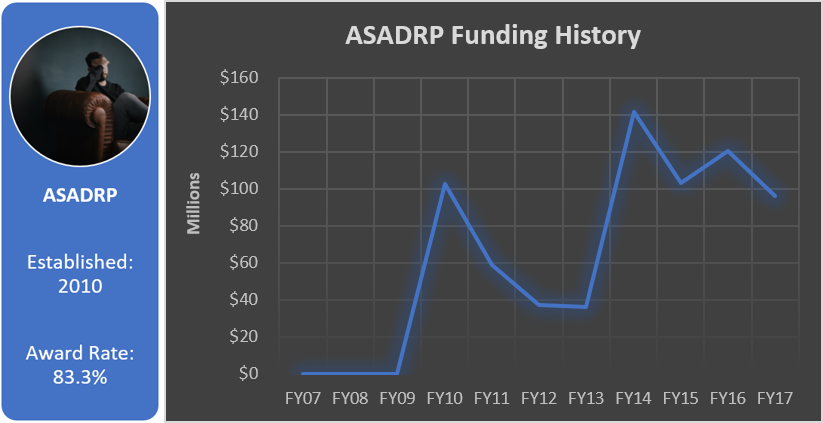
Total appropriated funds (2014-2017): $16.0 million
Additional DoD Programs for TBI Research
Army Research Laboratory (ARL)
The U.S. Army Research Laboratory (ARL) is the Army’s corporate laboratory and sole fundamental research laboratory. The ARL’s mission is to “Discover, innovate, and transition Science and Technology (S&T) to ensure dominant strategic land power.” The ARL has a history of occasionally releasing solicitations for TBI or TBI-related research.
Focus Areas
The current ARL BAA for Basic and Applied Scientific Research is accepting research proposals from April 2017 until March 2022. It includes the following TBI-related research topic:
- Sciences for Lethality and Protection – Humans in Extreme Ballistic Environments: The goal of this research is to provide a mechanism based understanding of the human response to blast (including TBI) and ballistic insults that will lead to advances in protections sciences and, ultimately, Army capabilities.
U.S. Army Medical Research and Materiel Command (USAMRMC)
The U.S. Army Medical Research and Materiel Command (USAMRMC) has several research programs which fund TBI-related research projects. These include the Combat Casualty Care Research Program (CCCRP), the Clinical and Rehabilitative Medicine Research Program (CRMRP), and the Military Operational Medicine Research Program (MOMRP). These major research areas are managed by Joint Program Committees (JPCs) consisting of DoD and non-DoD medical and military technical experts. The current USAMRMC Broad Agency Announcement (BAA) for Extramural Medical Research was released October 2017 and is open until September 2022.
Focus Areas
Combat Casualty Care Research Program (CCCRP)
The JPC-6/CCCRP supports research in combat casualty care (e.g., tactical care; basic and advanced life support; delayed care or prolonged field care, and enhanced triage capabilities), citing TBI as one of the principal causes of death among Service members who die within the first hour of wounding. Current CCCRP Research Areas of Interest involving TBI include:
- Research and development of technologies to diagnose and to limit the immediate, short-, and long-term impairments that follow TBI and spinal cord injury: Research specializing in “polytrauma” accounting for the impact of hemorrhagic shock and failure to oxygenate and/or ventilate as brain injury progresses is of interest to provide insight leading to improvements in clinical practice guidelines. Included in this area of interest are non-invasive or minimally invasive sensors or assays to rapidly diagnose the severity of brain and neurological injury within the battle area (or as close to it as possible) and drugs, biologics, or other agents to mitigate the progression of neurotrauma/secondary brain injury such as post-injury neural and immune cell overstimulation, inflammation, cell loss, and/or neurologic dysfunction.
Clinical and Rehabilitative Medicine Research Program (CRMRP)
The JPC-8/CRMRP focuses on the innovations required to reset our wounded Service members, both in terms of duty performance and quality of life. Current CRMRP Research Areas of Interest involving TBI include:
- Hearing Loss/Dysfunction, Balance Disorders, and Tinnitus: Seeks research efforts to support the development of strategies and technologies (including, but not limited to, medical devices, pharmaceuticals, rehabilitation strategies, and regenerative medicine-based approaches) to restore and/or rehabilitate patients with hearing loss/dysfunction, balance disorders, and/or tinnitus due to trauma (including TBI).
- TBI Rehabilitation: Seeks to develop, evaluate, and/or validate rehabilitation intervention strategies (e.g., multitask/dual-task) to address TBI-related sequelae. Interventions should remediate TBI-related deficits including, but not limited to, dizziness, cognitive dysfunction (e.g., attention, memory, or impaired executive function), or sensorimotor deficits (e.g., gait instability).
- Ecological Assessment: Aims to develop, evaluate, and/or validate return to duty (RTD) outcome measures following rehabilitation in patients with mild TBI. Outcome measures should be both ecologically valid (i.e., focused on military-specific tasks) and clinically feasible, with an aim to inform RTD/participation decisions in either an operational or garrison-based environment.
Military Operational Medicine Research Program (MOMRP)
The mission of JPC-5/MOMRP is to develop effective countermeasures against military-relevant stressors and to prevent physical and psychological injuries during training and operations in order to maximize the health, readiness, and performance of Service members and their families. Current MOMRP Research Areas of Interest involving TBI include:
- Psychological Health and Resilience: Research aimed at maximizing resilience and psychological health and decreasing PTSD, depression and anxiety disorders, suicide, and risk behaviors (e.g., substance abuse, anger/aggression, sexual harassment and assault, and interpersonal violence within the military). In addition, this research area may include development and validation of effective evidence-based training and prevention interventions for concussion/mild TBI.
What Now?
If your small business concern (SBC) is developing an innovative dual-use technology for TBI or in one of our other 2018 technology focus areas (cancer therapeutics, orthopaedic trauma tech, and sensors/internet of things), we may be able to assist by:
-
- Determining whether your technology is a capability interest to the DoD;
- Identifying non-dilutive funding opportunities that are available for your SBC to apply;
- Identifying DoD subject matter experts for collaboration via CRADAs;
- Prioritizing the non-dilutive funding application to pursue near-term, and most importantly why; and
- Assisting with DoD-specific narrative/terminology development for your submission package.
If you would like more information on how Tier Seven can assist your small business with identifying, connecting and collaborating via CRADAs with other DoD researchers, please contact us.
26 Dec
2017
