
Previously we wrote about Biomedical and Line (non-medical) Department of Defense (DoD) Technology Readiness Levels (TRLs) and medical countermeasure (MCM) TRLs and discussed how a technology may develop through each TRL stage. Here, we will provide additional details on how biomedical TRLs apply specifically to early discovery and development (i.e., TRLs 1-3) drug, biologic or vaccine decision points for new chemical entities (NCE).
DoD biomedical-related technologies require TRL definitions and descriptions that are appropriate to the technologies upon which they are based and that account for the statuses and regulations that govern their development and use. In recognition of these factors, the U.S. Army Medical Research and Materiel Command (USAMRMC) established definitions, descriptions, and processes in the context of military medical research and development and that incorporate Food and Drug Administration (FDA) statutory and regulatory requirements. These are provided in Appendix E of the Department of Defense Technology Readiness Assessment (TRA) Deskbook that was published in July 2009.
A frequently asked question is: “How do these biomedical TRLs relate to the FDA’s drug discovery and development process for drugs?” To begin to answer this broad question, we present a co-mingled listing of DoD biomedical TRLs; Biomedical Advanced Development Authority (BARDA) TRLs for MCM Products (Drugs and Biologics); and information on “Early Drug Discovery and Development Guidelines: For Academic Researchers, Collaborators, and Start-up Companies” by Strovel et al. in the “Assay Guidance Manual” from the National Center for Advancing Translational Sciences (NCATS). The NCATS guideline provides information on developing therapeutic hypotheses, target and pathway validation, proof-of-concept criteria and generalized cost analyses at various stages of early drug discovery. In the following discussion on TRLs focused on pharmaceuticals, we will incorporate the NCE discovery and development Decision Points presented in these guidelines in order to provide more precision in defining where a therapeutic candidate is in the biomedical TRL process. A “Decision Point” is the latest moment at which a predetermined course of action is initiated. Project advancement based on decision points balance the need to conserve scarce development resources with the requirement to develop the technology to a commercialization point as quickly as possible. Failure to meet the criteria listed for the following decision points will lead to a No-Go recommendation. The guideline also provides informative cost estimates and timelines (based on the costs and experience of the authors for developing oncology products) for these processes that can be refined based on your company’s requirements, capabilities, and type of product in development.
Biomedical TRLs (Pharmaceutical Focus)
TRL 1: Basic Principles Observed and Reported (DoD TRL); Review of Scientific Knowledge Base (DoD Biomedical & BARDA TRLs)
TRL 1 is the lowest level of technology readiness involving maintenance of scientific awareness and generation of the scientific and bioengineering knowledge base. Scientific findings are reviewed and assessed as a foundation for characterizing new technologies.

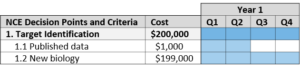
Target Identification
Target-based drug discovery begins with identifying the function of a possible therapeutic target and its role in the disease.

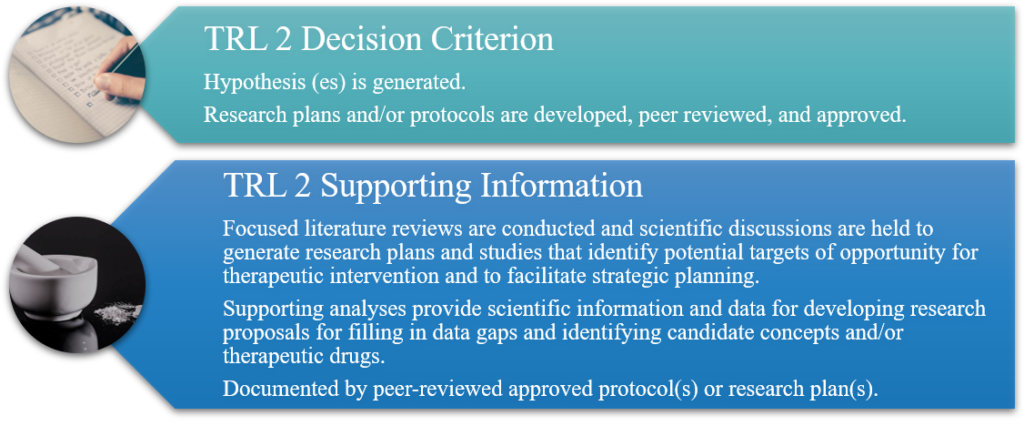
TRL 2: Technology Concept and/or Application Formulated (DoD TRL); Development of Hypotheses and Experimental Designs (DoD Biomedical & BARDA TRLs)
TRL 2 for pharmaceuticals involves intense intellectual focus on the problem, with generation of scientific “paper studies” that review and generate research ideas, hypotheses, and experimental designs for addressing the related scientific issues.
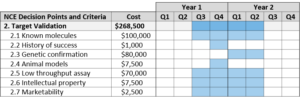
Target Validation
Target validation requires a demonstration that a molecular target is directly involved in a disease process, and that modulation of the target is likely to have a therapeutic effect.

TRL 3: Analytical and Experimental Critical Function and/or Characteristic Proof of Concept (DoD TRL); Target/Candidate Identification and Characterization of Preliminary Candidate(s) (DoD Biomedical and BARDA TRLs)
Basic research, data collection, and analysis begin in order to test the hypothesis, explore alternative concepts, and identify and evaluate technologies supporting drug development or critical technologies and components supporting candidate biologic/vaccine constructs research and eventual development of a candidate countermeasure.
Drugs: Initial synthesis of countermeasure candidate(s) and identification of their sites and mechanisms of action. Initial characterization of candidates in preclinical studies.
Biologics & Vaccines: Agent challenge studies are conducted to support models based on presumed battlefield conditions. Research-scale process initiation and evaluation is conducted, as are studies to identify site(s) and mechanism(s) of action, potential correlates of protection for vaccines, and initial physical/chemical characterization of constructs.

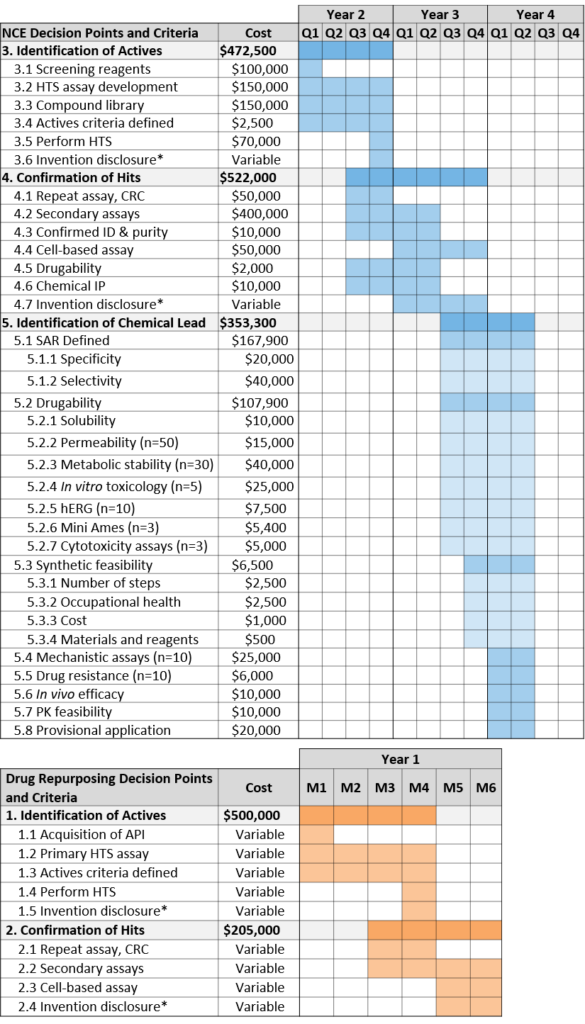
Identification of Actives
An active is defined as a molecule that shows significant biological activity in a validated screening assay that represents the disease biology and physiology. By satisfying the advancement criteria for identification of actives, the project team will begin to define new composition matter by linking a chemical structure to modulation of the target.
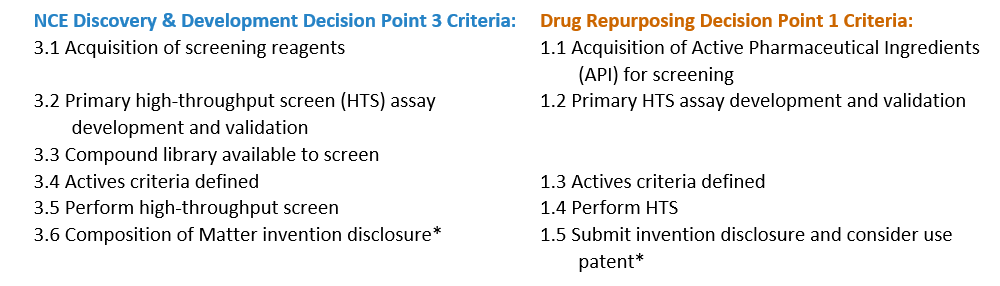
Confirmation of Hits
A hit is defined as consistent activity of a molecule (with confirmed purity and identity) in a biochemical and/or cell based secondary assay. Additionally, this is the point at which the project team will make an assessment of the molecular class of each of the hits.

Identification of Chemical Lead
A chemical lead is defined as a synthetically feasible, stable, and drug-like molecule active in primary and secondary assays with acceptable specificity and selectivity for the target. This requires definition of the Structure-Activity Relationship (SAR) as well as determination of synthetic feasibility and preliminary evidence of in vivo efficacy and target engagement. Projects at this stage may be eligible for Phase I SBIR.

In future articles, we will discuss additional TRLs and decision points for biomedical and MCM TRLs 4-9. Please let us know if this is helpful for your entities early drug discovery efforts, required edits based on your entities experiences, and if you are aware of additional resources to inform these important processes.
14 Aug
2018
